Services
   
Products   
Resources    |
DSB Scientific is actively engaged in physical chemistry and engineering research. Current physical chemistry projects include detailed descriptions of reaction pathways of energized systems, numerical modeling of detonations of atypical explosives and fluid dynamics simulations of transonic, supersonic and hypersonic projectiles.
Physical Chemistry Sample Results:
- Ab Initio Quantum Chemistry Calculation:
This image shows part of the computed z=0 (planar) energy surface for the
Argon-Acetone van der Waals complex. The calculation was done
using 0.1 angstrom steps, took over 90 wall-clock hours and
generated over 40 GB of data. About a half dozen custom utilities were written to generate and visualize this data. These data are used to help understand the physical chemistry of van der Waals bonding.
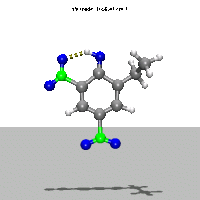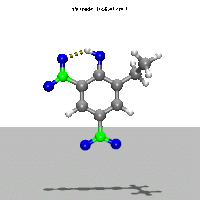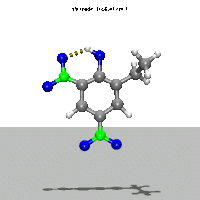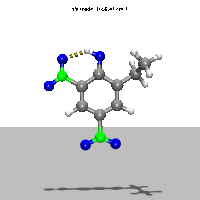
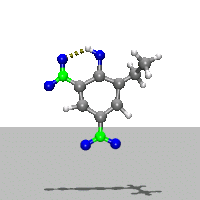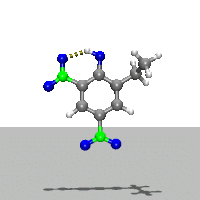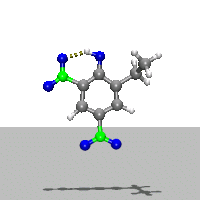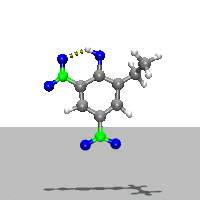
- Ab Initio Quantum Chemistry Calculation:
This image shows the computed structure of an ion-dipole complex that is possibly an intermediate in the decomposition reaction of ethyl-nitrophenol ions. Experimental data for such a complex is difficult or expensive to generate but are needed for detailed detonation modeling calculations.
- Spectroscopy:
Here is the computed infrared spectrum of a reactant gas phase ion. These data are then used to compute other properties using statistical mechanics methods.
- Educational Demonstrations in Statistical Mechanics:
Shown here is a series of screen shots from the MCGas program (just below the 'boiling point'). Calculations like these can be used to explore phase changes such as in a physical chemistry laboratory course.
- Fluid Dynamics Calculations:
These results of fluid dynamics calculations are part of DSB's research into the turbulent modeling of supersonic projectile drag functions.
This image shows the flow field as developed after
11 milliseconds for a NACA0012 airfoil at a five degree angle of
attack. The free stream velocity is Mach 1.5.
This image shows the flow field developing for an elliptical projectile at Mach 2.5 (angle of attack=0 degrees).
|