



Next: 8.1.3 What Mass Does
Up: 8.1 Isotope Peaks
Previous: 8.1.1 What are Isotope
Contents
The of the
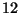 C atom is defined to be exactly
12 amu. This means there are an infinite number of significant figures
in the mass of carbon-12. The mass of C is 12.011 amu. What? Is it
12.(infinite 0's) amu or is it 12.011 amu?
C atom is defined to be exactly
12 amu. This means there are an infinite number of significant figures
in the mass of carbon-12. The mass of C is 12.011 amu. What? Is it
12.(infinite 0's) amu or is it 12.011 amu?
- The atomic mass scale is defined by the mass of the
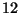 C
atom. Note that this is a specific isotope of carbon. All other
atomic masses are relative to the mass of this atom. In addition,
the mole is a number unit defined from the atomic mass scale as the
number of particles in exactly 12 g of carbon-12. The molar
mass of carbon-12 is defined to be exactly 12 g/mol.
C
atom. Note that this is a specific isotope of carbon. All other
atomic masses are relative to the mass of this atom. In addition,
the mole is a number unit defined from the atomic mass scale as the
number of particles in exactly 12 g of carbon-12. The molar
mass of carbon-12 is defined to be exactly 12 g/mol.
- What does the symbol C represent? Recall that there are three 'common'
isotopes of carbon:
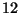 C,
C,
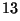 C and
C and
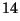 C.
The symbol C represent 'natural carbon,' which means a sample of carbon
that contains all three isotopes distributed in their natural relative
abundances.
C.
The symbol C represent 'natural carbon,' which means a sample of carbon
that contains all three isotopes distributed in their natural relative
abundances.
- The molar mass of natural carbon is the mass of one mole of natural
carbon. Taken relative to one mole of carbon-12, the molar mass of
natural carbon is experimentally found to be 12.011 g/mol. Further,
this mass is the average mass as calculated from
Using 98.9% for the natural abundance (probability) of carbon-12
and 1.1% for the probability of carbon-13, the average carbon mass
is calculated to be 12.011 amu.
- This applies to other elements as well.
Exercise: Consult a CRC Handbook of Chemistry and Physics or
a General Chemistry Text for the mass of carbon-13 and confirm the
calculation of average mass of carbon (Hint: the mass of carbon-13
is not exactly 13 amu).




Next: 8.1.3 What Mass Does
Up: 8.1 Isotope Peaks
Previous: 8.1.1 What are Isotope
Contents
John S. Riley, DSB Scientific Consulting
![]() C atom is defined to be exactly
12 amu. This means there are an infinite number of significant figures
in the mass of carbon-12. The mass of C is 12.011 amu. What? Is it
12.(infinite 0's) amu or is it 12.011 amu?
C atom is defined to be exactly
12 amu. This means there are an infinite number of significant figures
in the mass of carbon-12. The mass of C is 12.011 amu. What? Is it
12.(infinite 0's) amu or is it 12.011 amu?