



Next: 4.2.3 Retention Index and
Up: 4.2 How a GC
Previous: 4.2.1 Basic Chromatography Theory
Contents
The nature of the interaction of a solute with a given stationary
phase is generally van der Waals forces. It would be quite cumbersome
to constantly refer to these interactions, their functional form and
magnitude, directly. It is more succinct to communicate the interaction
via parameters based on the retention a phase causes for a given solute.




Next: 4.2.3 Retention Index and
Up: 4.2 How a GC
Previous: 4.2.1 Basic Chromatography Theory
Contents
John S. Riley, DSB Scientific Consulting
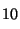 H
H
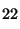 =
1000, the RI of n-C
=
1000, the RI of n-C
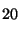 H
H
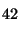 = 2000, etc.
That is, the RI is defined as 100*n where n is the number of carbons
in a straight chain hydrocarbon. {insert figure}
= 2000, etc.
That is, the RI is defined as 100*n where n is the number of carbons
in a straight chain hydrocarbon. {insert figure}