



Next: 2.1.5 Spectroscopy
Up: 2.1 General Theory of
Previous: 2.1.3 Polyatomic Oscillators
Contents
2.1.4 Allowed transitions in IR spectroscopy
In order for a mode to have an allowed
IR transition, the mode must involve a change in the electric dipole
moment of the molecule. Stated another way, if the mode is associated
with a time varying dipole, the mode can interact with infrared electromagnetic
energy to produce a vibrational energy transition. A permanent dipole
moment is not required. Consider carbon dioxide, a molecule
with no permanent electric dipole moment. (Here, the phrase 'permanent
dipole moment' refers to the dipole moment at the equilibrium geometry
of an hypothetically non-vibrating molecule.
- There are 3 atoms (
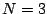 ) and the molecule in its equilibrium geometry
is linear. There are, therefore, 4 vibrational modes.
) and the molecule in its equilibrium geometry
is linear. There are, therefore, 4 vibrational modes.
- The hypothetical non-oscillating molecule has no permanent dipole
moment since the two opposed individual dipole vectors cancel. {insert
figure}
- Since the dipole moment does not change during symmetric stretching,
the symmetric stretch mode is inactive. {insert figure}
- Three modes do cause the dipole moment to change: two degenerate symmetric
bends (in plane and out-of-plane) {insert figure} and the asymmetric
stretch. {insert figure} These modes, therefore, are infrared active.




Next: 2.1.5 Spectroscopy
Up: 2.1 General Theory of
Previous: 2.1.3 Polyatomic Oscillators
Contents
John S. Riley, DSB Scientific Consulting